On August 11th, 2025, the European Union published the Regulation (EU) 2025/1731 in its official gazette, which revised the restricted list of carcinogenic, mutagenic and reproductive toxic (CMR) substances in Annex XVII of REACH Regulation. This revision adds 16 Category 1B CMR substances and one exemption clause, which will take effect on September 1, 2025.
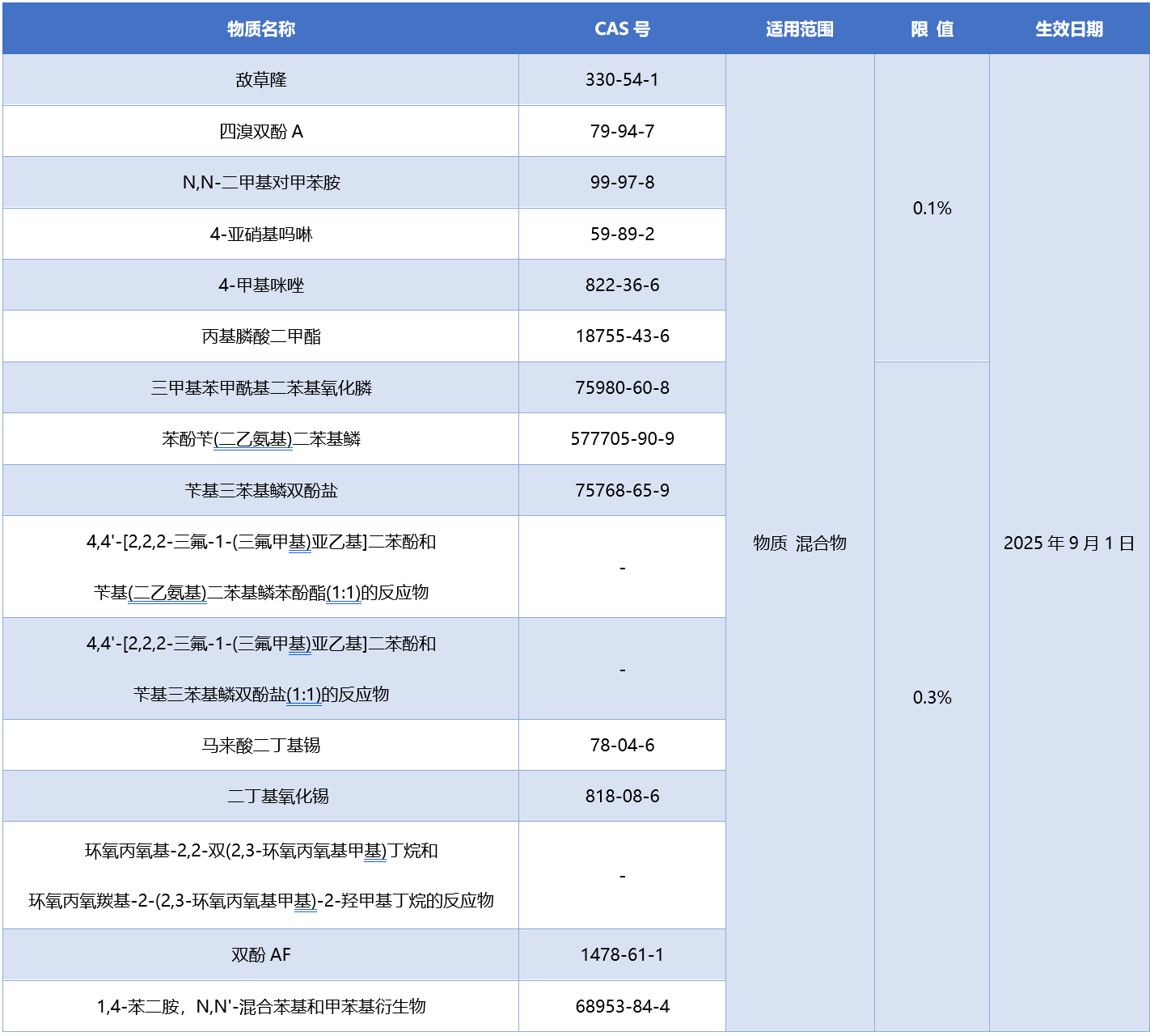
Added 16 items of information about Class 1B CMR substances
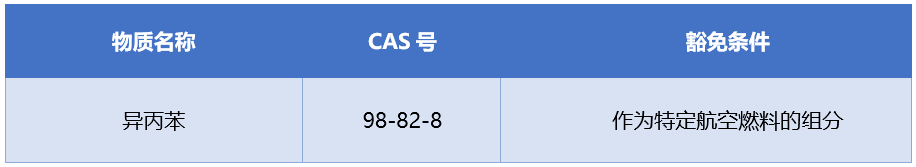
Add one exemption clause
Warm tips
With the continuous updating of Appendix XVII of REACH regulations, enterprises are faced with more and more control requirements. ZRLK suggests relevant enterprises to improve their risk awareness of products, pay close attention to the update trends of REACH regulations in time, adjust production strategies, ensure that products containing restricted substances are exported to meet the latest regulatory requirements and avoid trade risks. Our company has a professional technical team and rich experience in product testing, which can help you easily understand whether the products are safe and compliant. If you need it, please feel free to contact us, and our engineers will serve you at the first time!